Oxford UK, 15 June 2019 – Celleron Therapeutics, the UK-based company developing personalised medicines for cancer patients, announced today that its Founding Director and Chief Medical Officer Professor David Kerr presented at the 15th Shanghai International Colorectal Cancer Symposium.
Professor Kerr began by introducing CXD101: Celleron Therapeutics’ next generation epigenetic immune-regulator, which represents a class of drug that kills cancer cells by blocking certain vital functions involved in gene expression (histone–deacetylase [HDAC] inhibition) and reactivates the patient’s immune system so that cancer cells can no longer evade immune recognition.
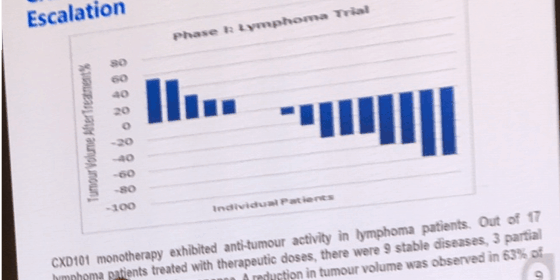
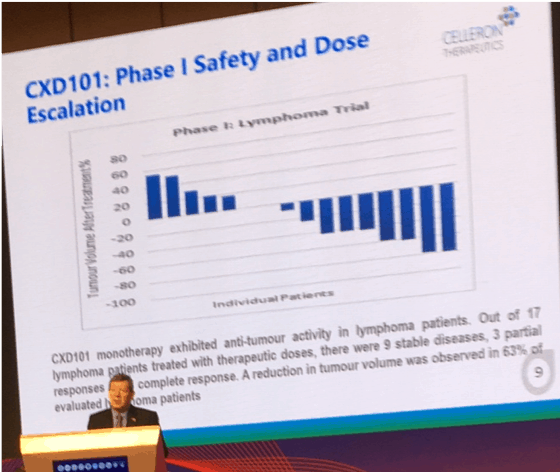
In a Phase I clinical trial, CXD101 was shown to be effective as a single agent in shrinking a range of tumour types, in patients who were resistant to, or relapsed following previous lines of chemotherapy.
He then went on to describe the CAROSELL clinical trial that Celleron Therapeutics is currently conducting, combining Celleron’s CXD101 with nivolumab, for microsatellite stable colorectal cancer.
Colorectal cancer is the second most common tumour type in women, and the third most common in men, globally. The approximate 5-year survival rate for colorectal cancer patients in the United States (all stages included) is 65%. Survival is inversely related to stage: approximate 5-year survival rates are 95% for patients with stage I disease, 60% for those with Stage III disease, and 10% for those with Stage IV (metastatic) disease.
Surgery is indicated for localized disease, whilst chemotherapy has been the standard management for patients with metastatic colorectal cancer. Two agents have been approved for third line management of advanced colorectal cancer, namely regorafenib and Trifluridine-tipiracil hydrochloride (Lonsurf).
A subset (5%) of colorectal cancers is characterized with deficient DNA mismatch repair (dMMR or microsatellite instability, MSI). These tumours tend to have a high expression of checkpoint proteins (PD-1 and PD-L1), which interfere with the body’s normal anti-tumour T-cell response. By disabling these proteins, checkpoint inhibitors such as nivolumab allow the immune system to function properly, and T-cells to kill tumour cells.
However, for the greater majority of patients with a normal Mismatch Repair proficient expression, the microsatellite phenotype is stable (MSS), antigen presentation is believed to be much decreased, and the tumour is thus resistant to checkpoint inhibition. Most MSS patients will ultimately relapse or become resistant to chemotherapy. Thus, there remains a very significant unmet clinical need to find novel agents, singly and/or in combination, for the treatment of these late-stage patients.
Celleron’s Phase II clinical trial (CAROSELL Study) of the effect of CXD101 in combination with nivolumab in MSS colorectal cancer is important because this tumour type typically does not respond to IO agents alone. Professor Kerr commented that this clinical trial strategy rests on compelling pre-clinical results which provide novel insights into how CXD101 and IO drugs work together to re-engage recognition of tumours by the immune system.
Professor Kerr is Honorary Professor of Oncology at Sichuan, Suchow, Xiamen and the 2nd Military University in CHINA and this conference will further develop the already strong links that Celleron Therapeutics enjoys across China and the region.
Professor David Kerr, Chief Medical Officer of Celleron Therapeutics, commented:
“It is a privilege and honour to present in Shanghai at this international symposium. Colorectal cancer remains clinically unmet and it is very important that we use forefront research to help design effective therapies”.
Professor Nick La Thangue, Chief Executive and Founder of Celleron Therapeutics and Professor of Cancer Biology in the Department of Oncology at Oxford University, commented:
“The Presence of our CMO Professor Kerr at this high-profile meeting underscores the global interest in Celleron Therapeutics’ clinical strategy, particularly in China. We look forward to strengthening our presence in the region”.
NOTES:
About Celleron Therapeutics
Celleron Therapeutics is a biopharma advancing a clinical and pre-clinical pipeline of precision therapies for different cancer indications. The company is a spin-out from Oxford University and located on the Oxford Science Park, UK. Celleron Therapeutics has built a proprietary platform around epigenetic control and immune modulation, providing its drugs with a two-pronged attack on cancer. Celleron’s approach seeks to align the right drug with the right patient enabling a personalised approach to cancer therapy.
Celleron Therapeutics’ focus is on those cancers where there is still an unmet need for long-term disease control. It is hoped that not only will patients volunteering for our clinical trials benefit directly, but the results from these studies will ultimately allow the general use of more effective, safer medicines. Our goal is not only to treat cancer but improve quality of life during therapy by reduction of side effects.
Celleron has a global license partnership with Astra Zeneca and is also initiating new trials in China. The company secured investment in 2016 from a consortium of South Korean investors.
For more information see www.cellerontherapeutics.com
About CXD101
CXD101 is Celleron Therapeutics’ next generation epigenetic immune-regulator representing a class of drug that kills cancer cells by blocking certain vital functions involved in gene expression (histone–deacetylase [HDAC] inhibitor) and reactivates the patient’s immune system so that cancer cells can no longer evade immune recognition.
The European Medicines Agency (EMA) has previously granted CXD101 Orphan Drug Designation as single agent therapy, based upon early-phase trial efficacy seen in relapsed or refractory Peripheral T-Cell Lymphoma (PTCL) patients. A PTCL Phase II trial is scheduled to start late 2019 in China.
Celleron Therapeutics have an ongoing Phase II clinical trial investigating the effectiveness of CXD101 in combination with an immune oncology agent, against a type of colorectal cancer (microsatellite stable) which typically does not respond to IO agents alone (CAROSELL Study). The clinical trial strategy rests on compelling pre-clinical results which provide novel insights into how CXD101 and IO drugs work together to re-engage recognition of tumours by the immune system. The trial will also allow exploration of a range of new biomarkers to help select those patients likely to benefit most from combination therapy.
Celleron Therapeutics is also exclusively financing an investigator-led Phase
Ib/II clinical trial in diffuse large B-cell lymphoma (DLBCL) patients who have failed on chemotherapy, called the PLACARD Study. Study subjects would receive and CXD101 and an undisclosed immune-oncology drug together. The immune-oncology drug will be provided to the Investigator by a large undisclosed pharma manufacturer.
Please download the press release here