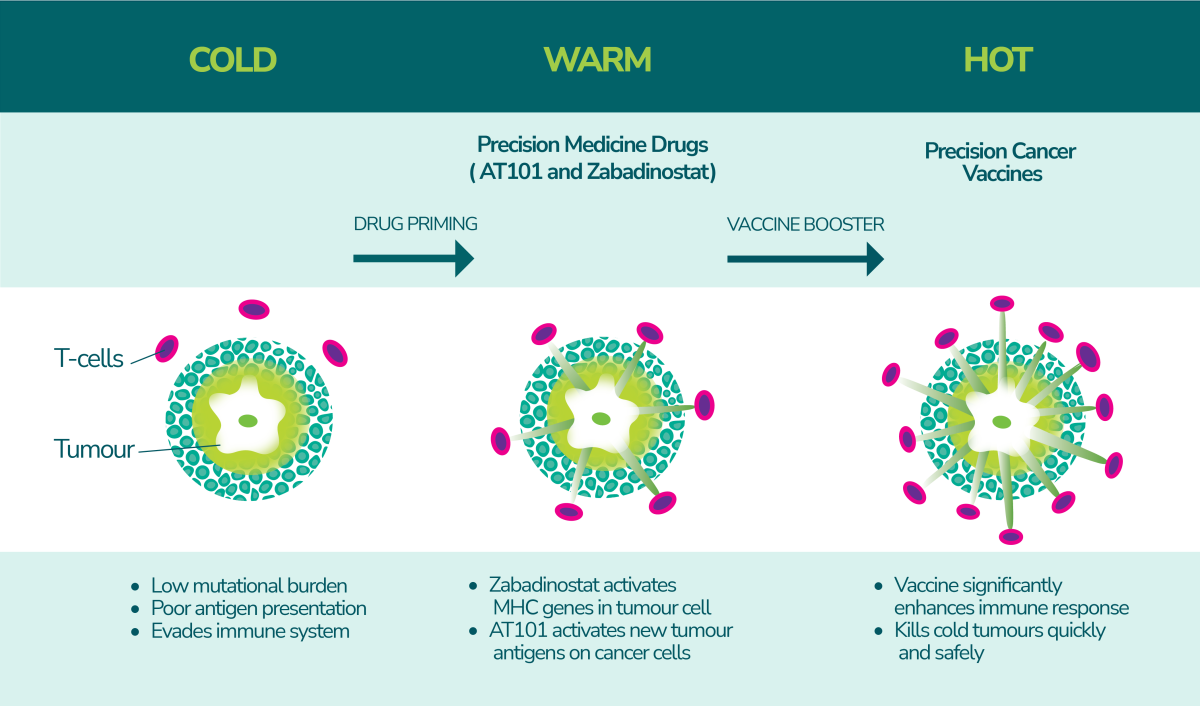
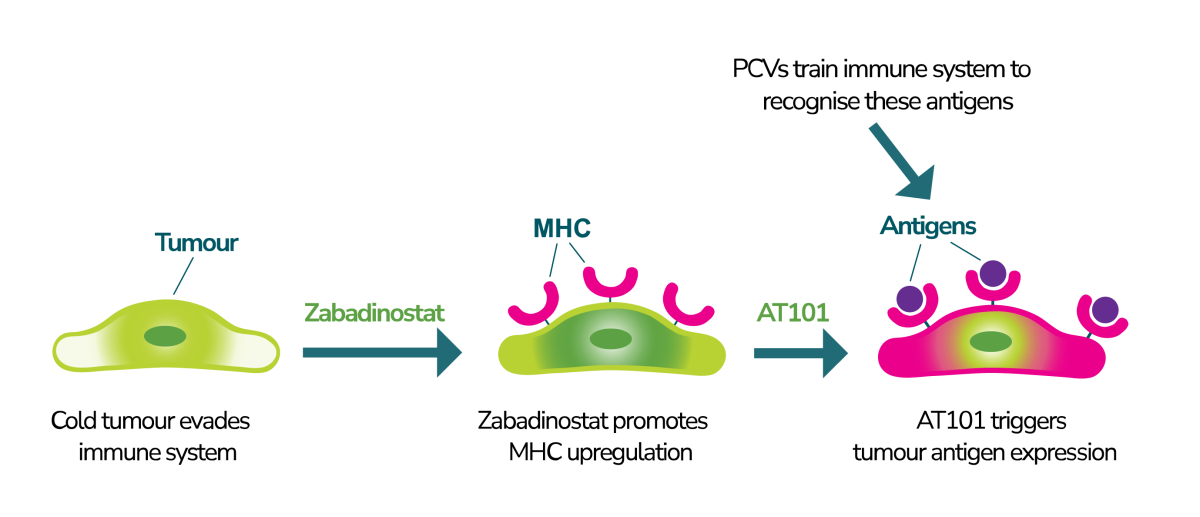
Zabadinostat is a small molecule immuno-modulator designed to enhance the immune system’s ability to recognise and destroy cancer cells. It works by modulating the expression of tumour antigens on cancer cells, making them more visible to the immune system. By boosting the activity of natural killer (NK) cells and T-cells, zabadinostat activates the immune system’s primary defenders to eliminate cancer cells.
By improving the immune system’s ability to identify and target tumours, zabadinostat offers a potentially safer and more effective alternative to traditional chemotherapy, which often comes with severe side effects and limited efficacy, especially in the treatment of advanced cancers.
Clinical Trials (Completed)
Phase I Study of Zabadinostat (CXD101)
A Phase I study explored the safety, tolerability, and pharmacokinetics of zabadinostat (CXD101) in patients with advanced solid tumours or recurrent/refractory lymphoma. The study identified the maximum tolerated dose and found that zabadinostat showed encouraging activity in patients with lymphoma, particularly those with Hodgkin lymphoma, T-cell lymphoma, and follicular lymphoma. Several patients experienced tumour reduction, with responses mainly observed in those with lymphoma. The treatment was generally well-tolerated, with common side effects including fatigue, nausea, and reversible cytopenia.
For more information, please visit https://pubmed.ncbi.nlm.nih.gov/30332497/
Phase II Trial: CXD101 and Nivolumab in Metastatic Microsatellite-Stable Colorectal Cancer (CAROSELL)
A Phase II study assessed the combination of zabadinostat (CXD101) and nivolumab in heavily pre-treated patients with metastatic microsatellite-stable (MSS) colorectal cancer, a form of cancer that typically does not respond to immune checkpoint inhibitors. The combination therapy was well tolerated, with the most common side effects being neutropenia and anaemia. Notably, immune-related adverse reactions commonly associated with checkpoint inhibitors were rare. The study showed encouraging efficacy, with several patients achieving stable disease or partial responses. The findings support further exploration of this combination therapy for MSS colorectal cancer.
For more information, please visit https://pubmed.ncbi.nlm.nih.gov/36327756/
Clinical Trials (Ongoing)
Phase II Study: Zabadinostat and Anti-PD-1 Therapy in Hepatocellular Carcinoma (HACCA)
An ongoing Phase II study, in partnership with the Chinese University of Hong Kong (CUHK), is assessing the combination of zabadinostat (CXD101) and geptanolimab, an anti-PD-1 therapy, in patients with hepatocellular carcinoma (HCC) who have developed resistance to prior immune checkpoint inhibitor (ICI) treatments. The combination therapy has been well tolerated, with the most common side effects being neutropenia and anaemia. Encouraging efficacy has been observed, with some patients achieving disease stabilisation. These findings support the continued exploration of this combination approach as a potential treatment for ICI-resistant HCC.
For more information, please visit https://clinicaltrials.gov/study/NCT05873244?intr=CXD101&rank=4
Clinical Trials (Planned)
Zabadinostat Combinations
IngenOx is planning to conduct a pilot study to explore the safety and immunological endpoints for the combination use of zabadinostat (CXD101) with other agents. The study will assess the potential of combining these therapies in treating advanced cancers, with a focus on immune response and tolerability. This will provide valuable insights into the safety profile and immunological impact of this combination approach, supporting further clinical development.
